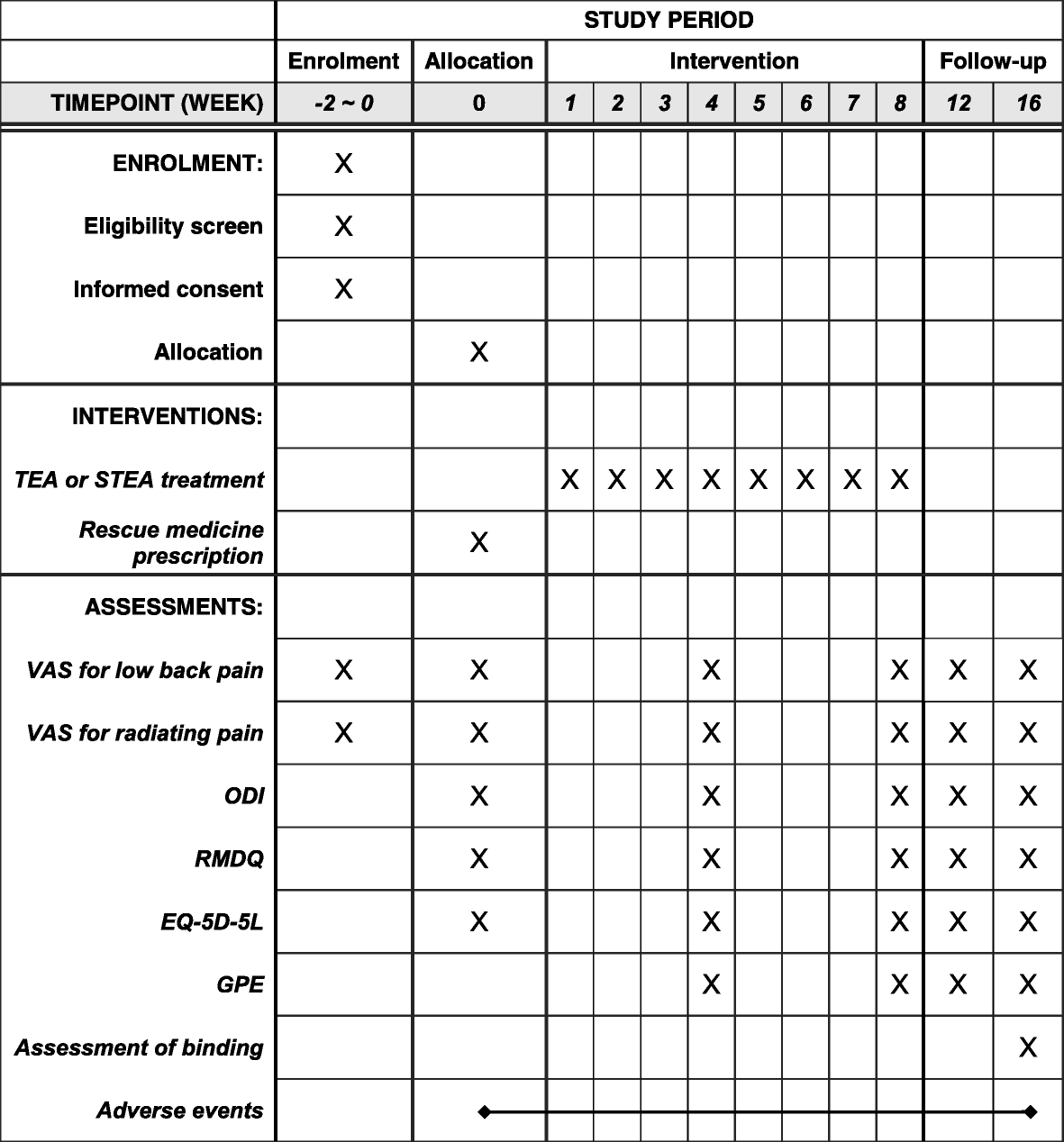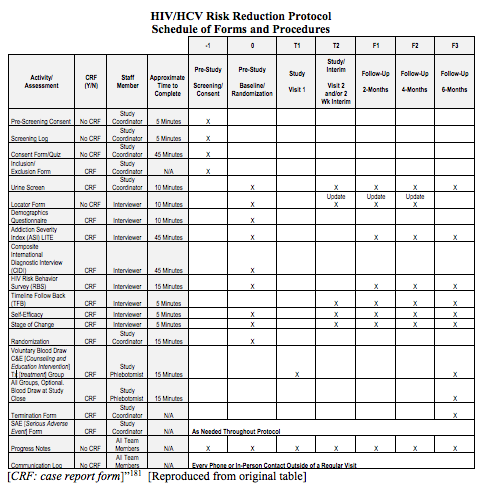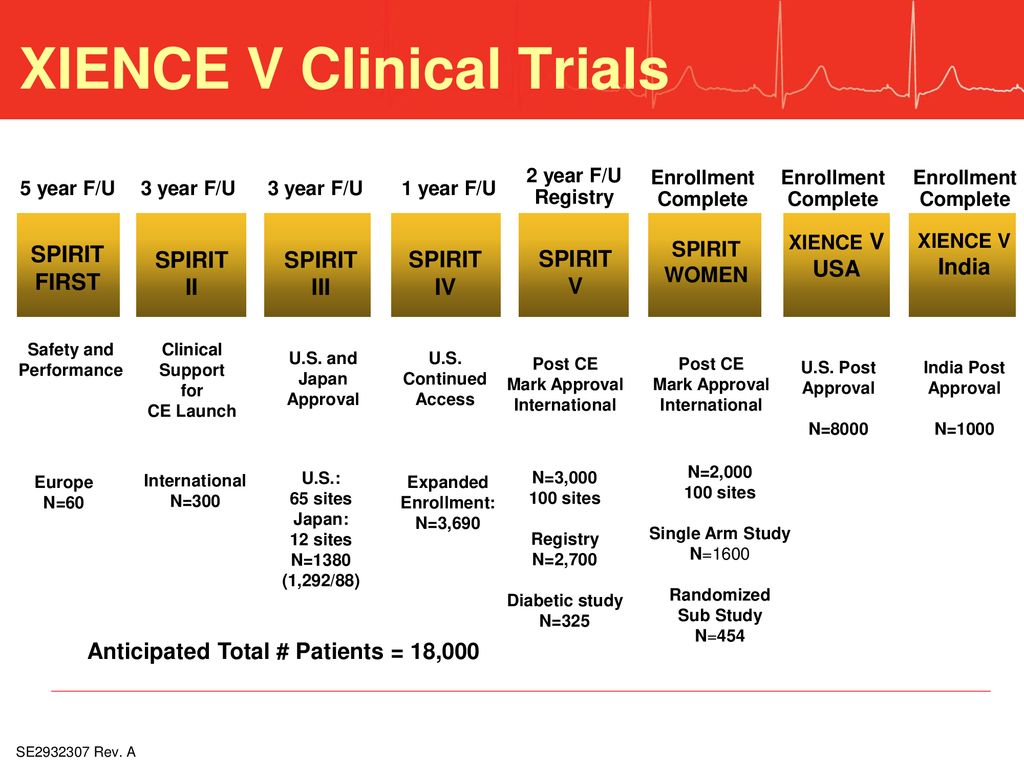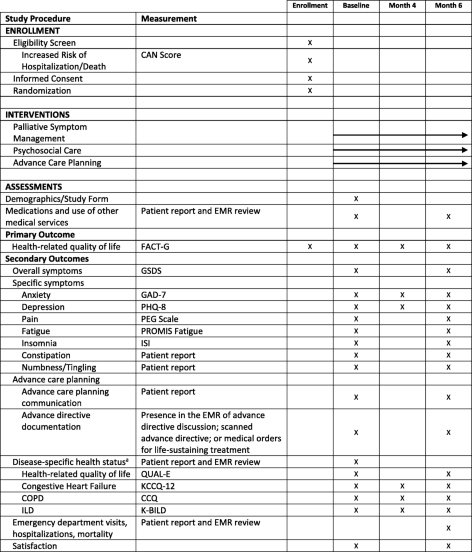
Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial | Trials | Full Text
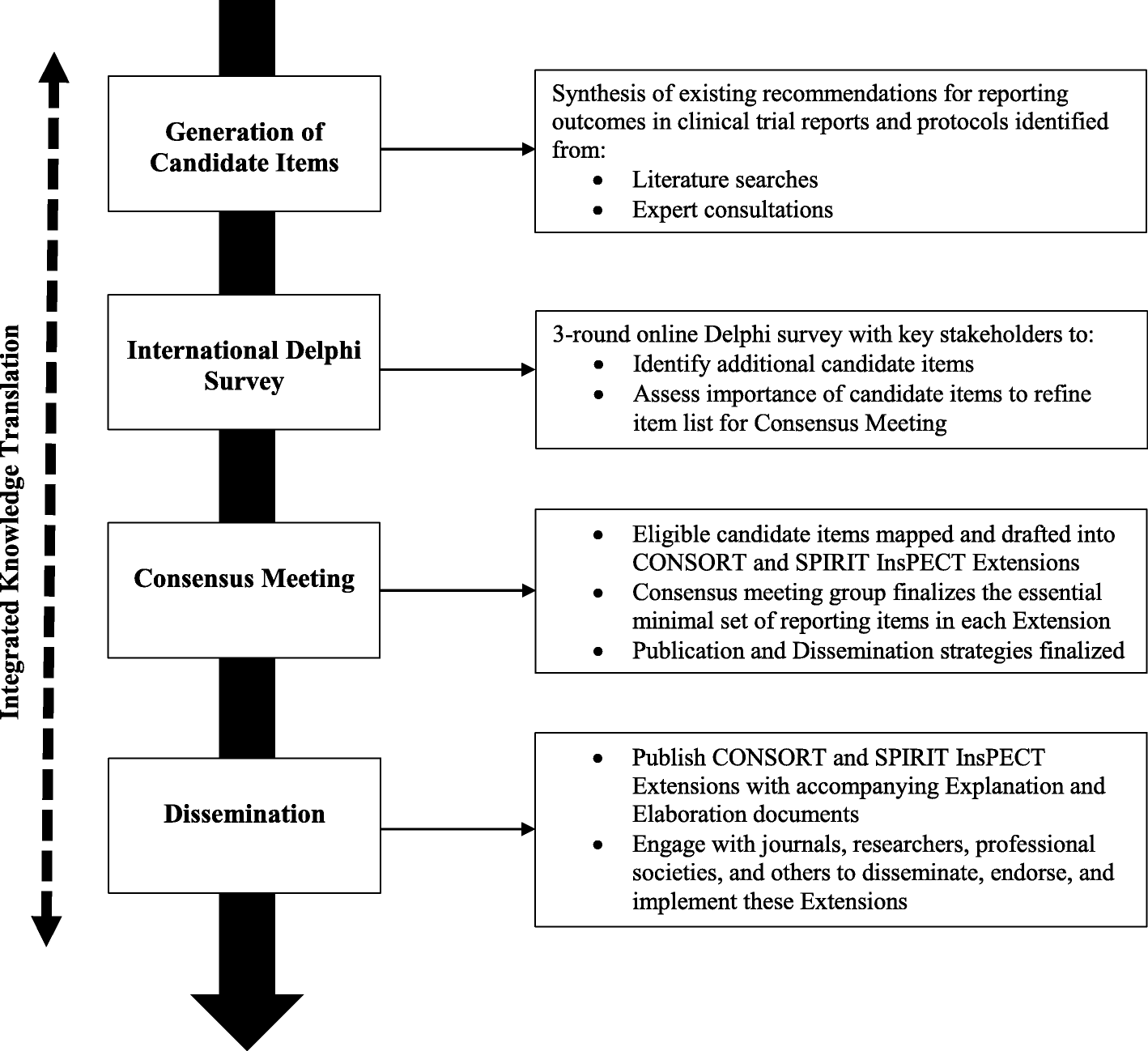
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Guidelines for cellular and molecular pathology content in clinical trial protocols: the SPIRIT-Path extension - The Lancet Oncology
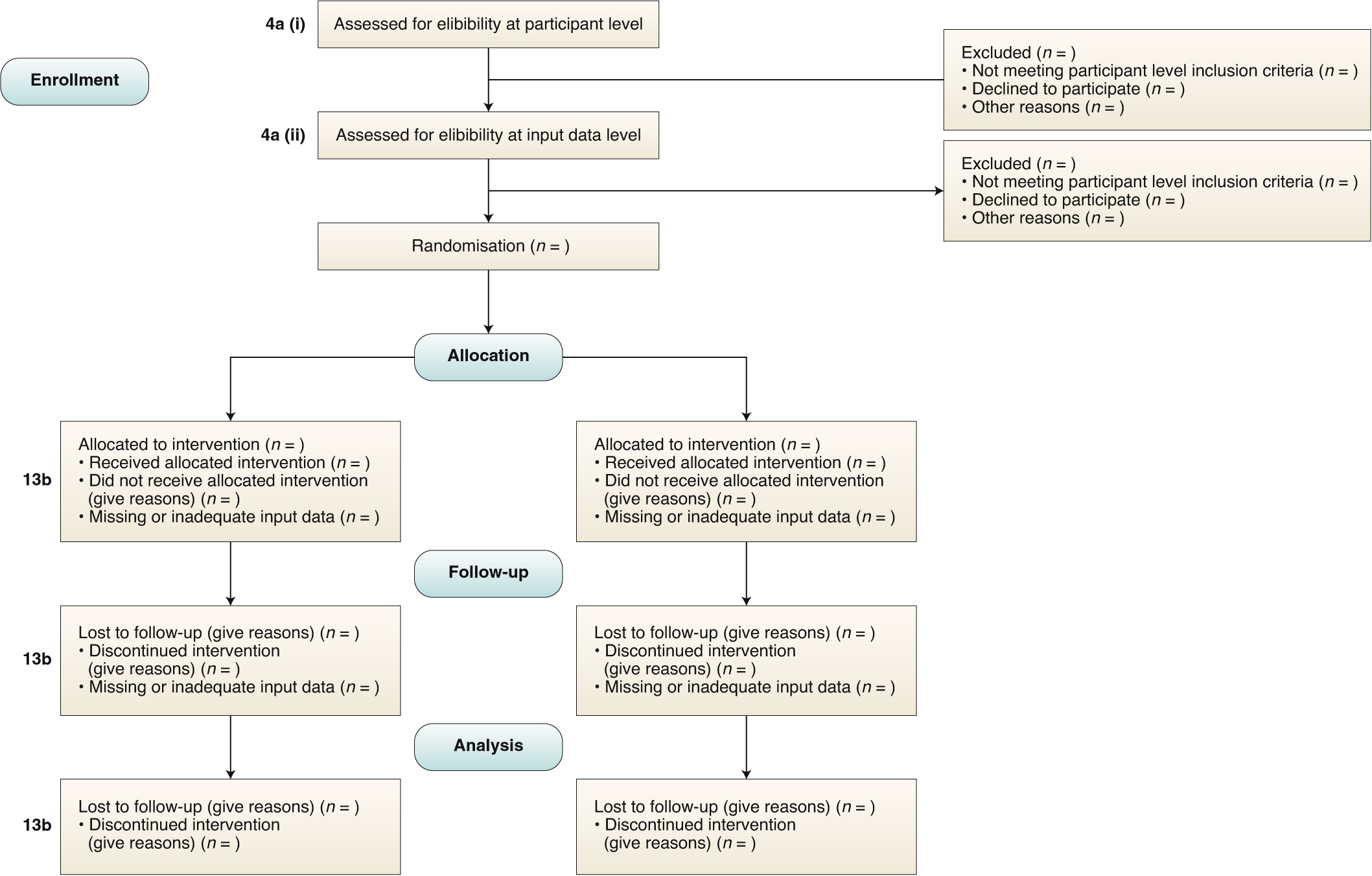
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine

SPIRIT diagram. The figure details the timing of enrollment activities,... | Download Scientific Diagram

Table 1 from SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar
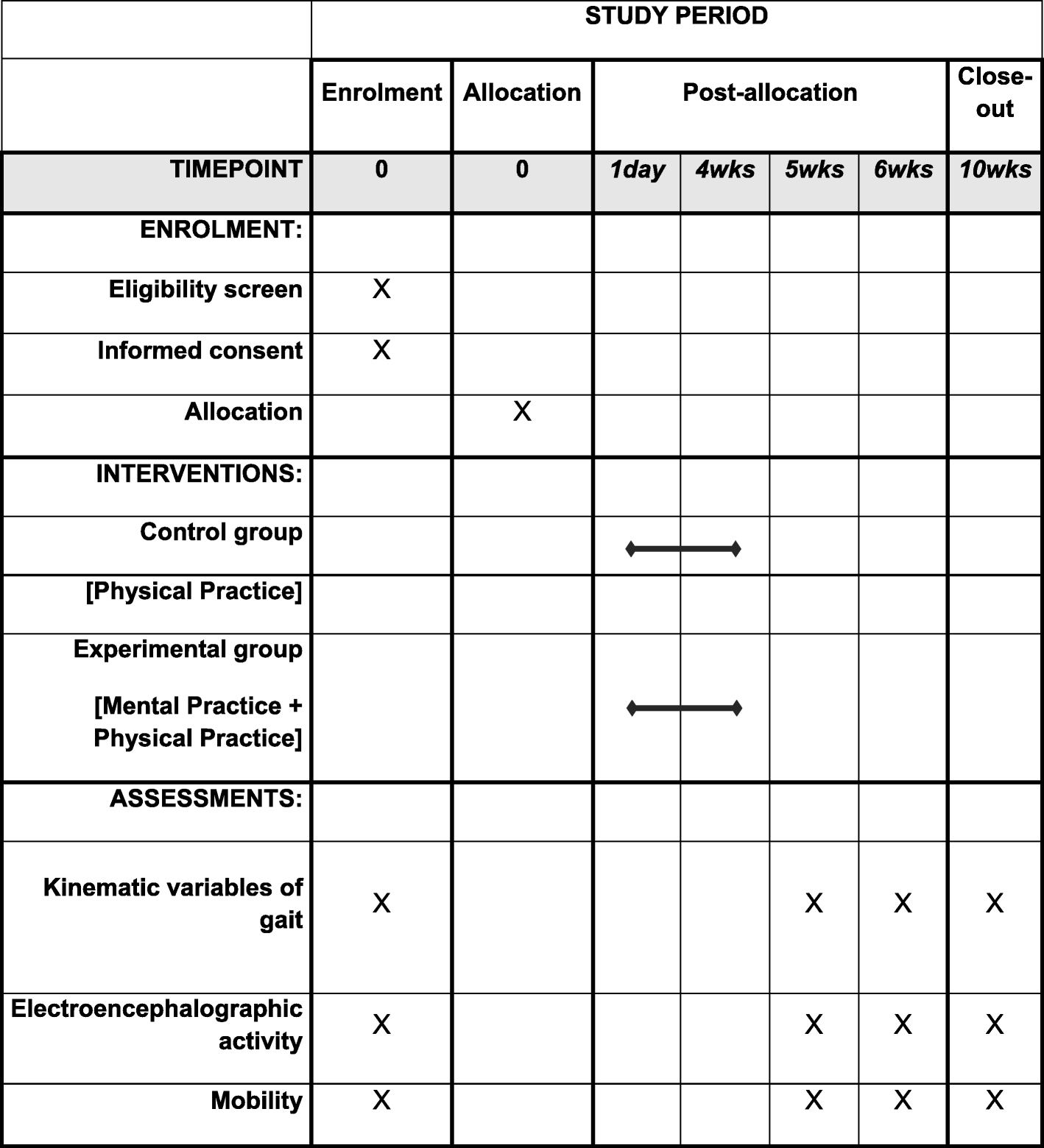
Effects of motor imagery training of Parkinson's disease: a protocol for a randomized clinical trial | Trials | Full Text

SPIRIT 2013 Checklist: Recommended items to address in a clinical trial... | Download Scientific Diagram

PDF) SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials | Trish Groves - Academia.edu
Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial ProtocolsThe SPIRIT-PRO Extension

Table 2 from SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - ScienceDirect

Hugh Harvey on Twitter: "Boom! Two sets for guidance for interventional clinical trial protocols (SPIRIT) and interventional clinical trial reports (CONSORT) involving AI. Cross-published in 3 major journals! Thanks to @EQUATORNetwork funded