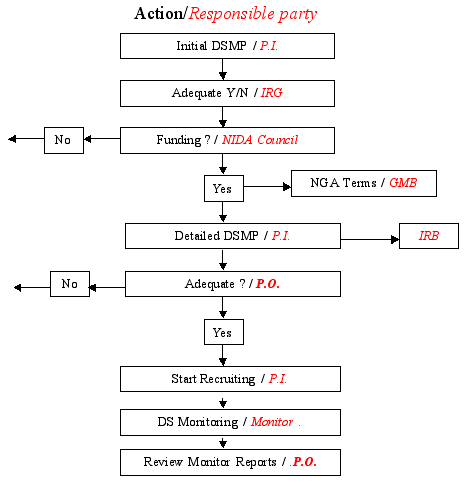
Guidelines for Developing a Data and Safety Monitoring Plan | National Institute on Drug Abuse (NIDA)

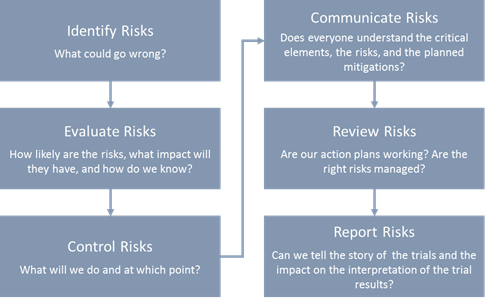
Risk Management – Clinical Trial Medical Monitoring Plan | Online Clinical Research Courses In India

Risk Management – Clinical Trial Medical Monitoring Plan | Online Clinical Research Courses In India

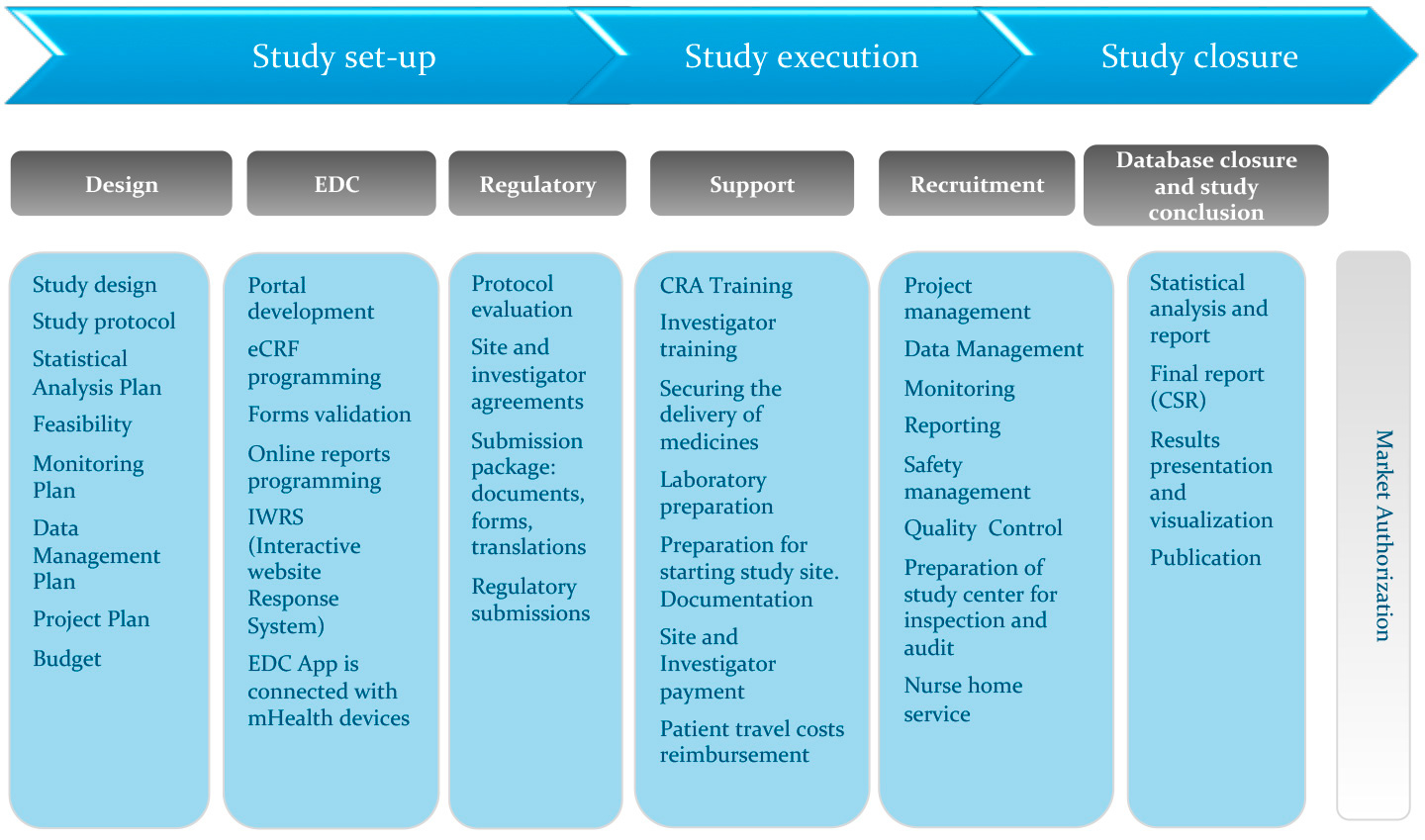
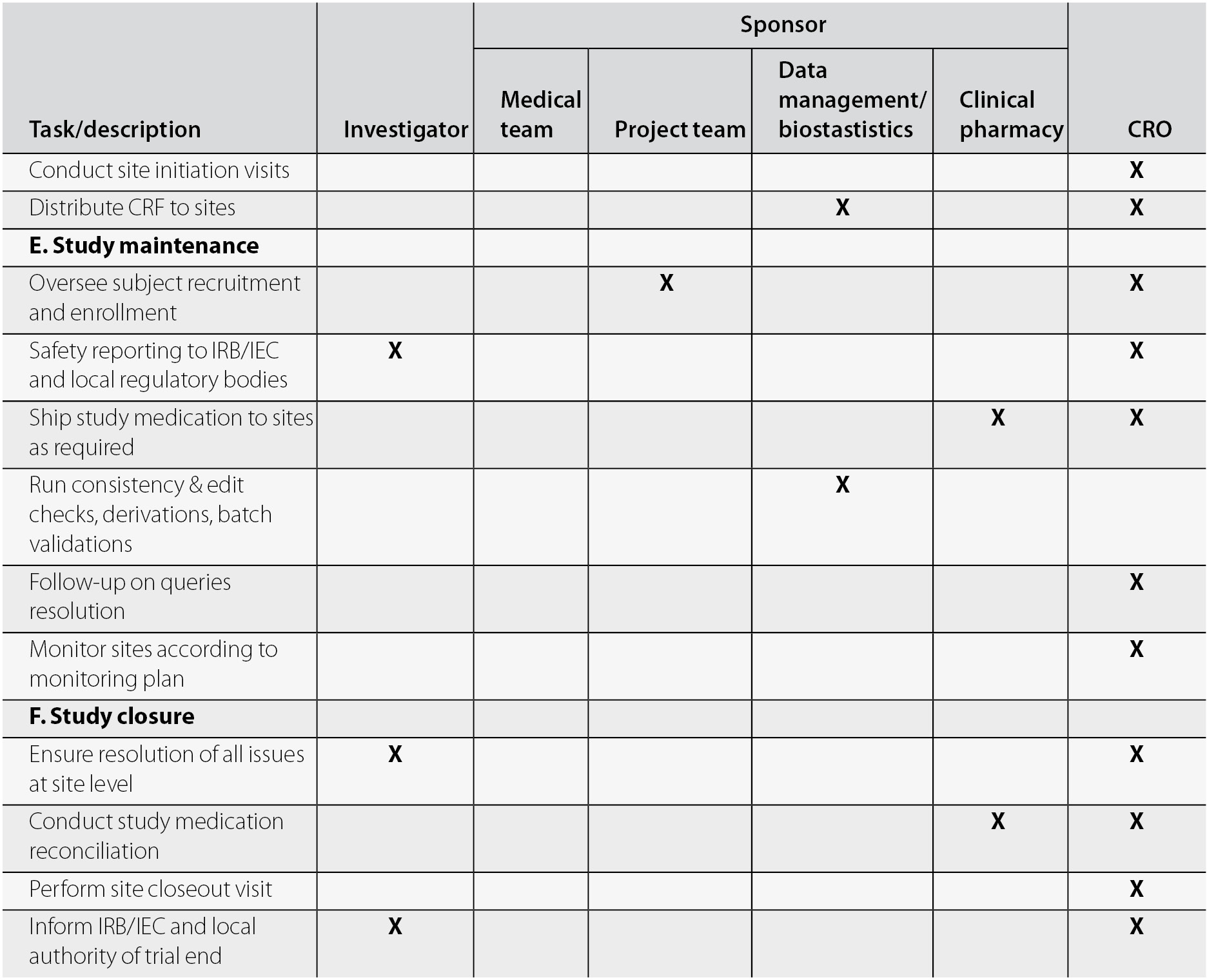
An international, full-service CRO that specializes in conducting and managing clinical trials. - PDF Free Download

Safety Management Plan – Clinical Trial Medical Monitoring Plan | Online Clinical Research Courses In India

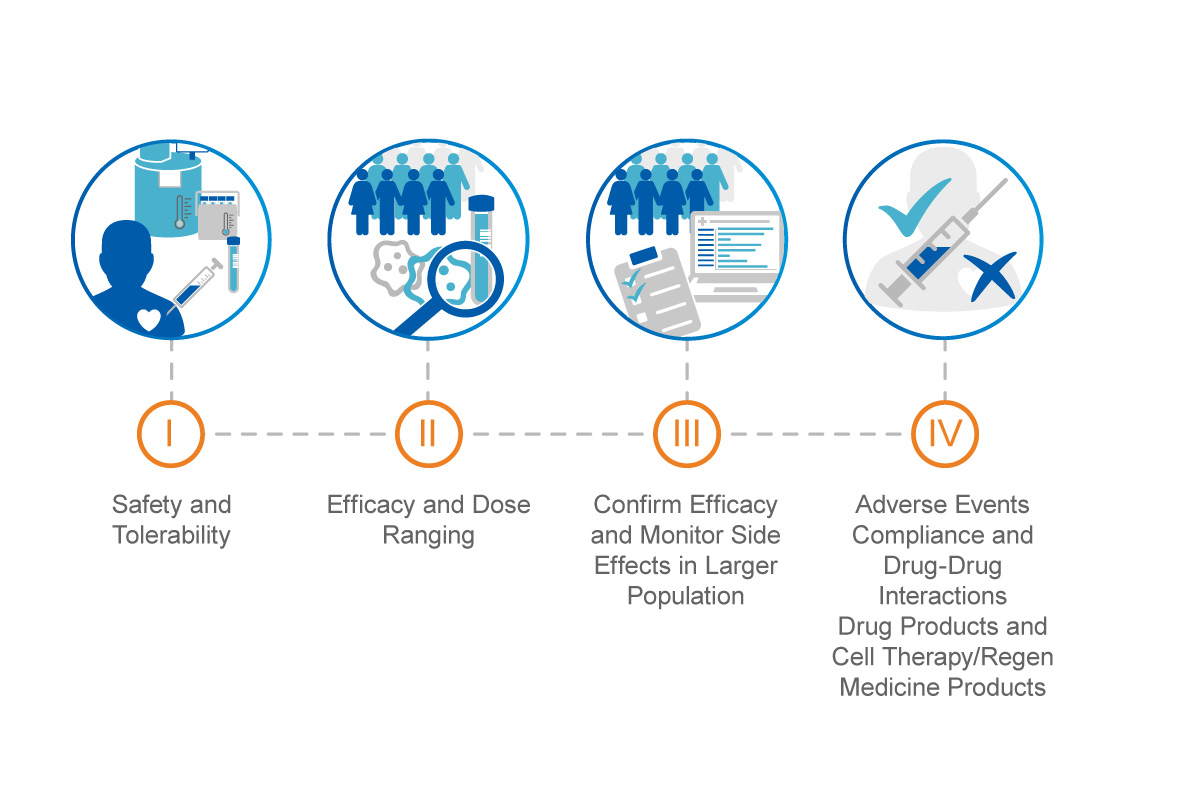
Phase I dose-escalation single centre clinical trial to evaluate the safety of infusion of memory T cells as adoptive therapy in COVID-19 (RELEASE) - eClinicalMedicine

WHAT IS THE SAFETY MANAGEMENT PLAN? - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal







d9a3b07696026559b82aff0000ccbde2.jpg?sfvrsn=b9a1d5cd_0)