Leo Pharma Enters into a Sub-Licensing Agreement with Bausch Health to Develop and Commercialize Brodalumab

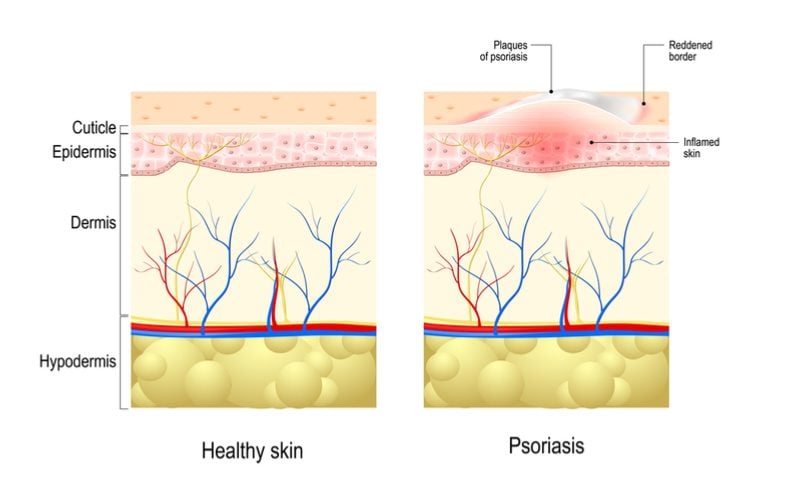
Brodalumab for the treatment of moderate-to-severe psoriasis: case series and literature review. - Abstract - Europe PMC

Immunogenicity and skin clearance recapture in clinical studies of brodalumab - Journal of the American Academy of Dermatology

Vuoi portare con me una crema per la psoriasi in aereo per la Cina - ragionevole? | Travelplansinmomhands

PDF) Complete clearance and psoriasis area and severity index response for brodalumab and ustekinumab in AMAGINE‐2 and ‐3

FDA panel unanimously backs Valeant's suicide-linked psoriasis med, but serious competition awaits | Fierce Pharma

PDF) Brodalumab for the treatment of moderate-to-severe psoriasis: case series and literature review

Improved Plaque Psoriasis Symptoms and Satisfaction With Brodalumab Therapy Reported - Dermatology Advisor











![Top 10 Plaque Psoriasis Clinical Trials [2022 Studies] | Power Top 10 Plaque Psoriasis Clinical Trials [2022 Studies] | Power](https://pwr-og-img.withpower.com/Apply%20to%20this%20Phase%203%20clinical%20trial%20treating%20Enterocolitis,%20Necrotizing,%20Necrotizing%20Enterocolitis%20(NEC),%20Enterocolitis:%0A%0ATreatment%20for%20Enterocolitis.png?md=1)